What Makes a Molecule Polar Apex
A molecule with polar bonds will be polar regardless of shape. THIS SET IS OFTEN IN FOLDERS WITH.
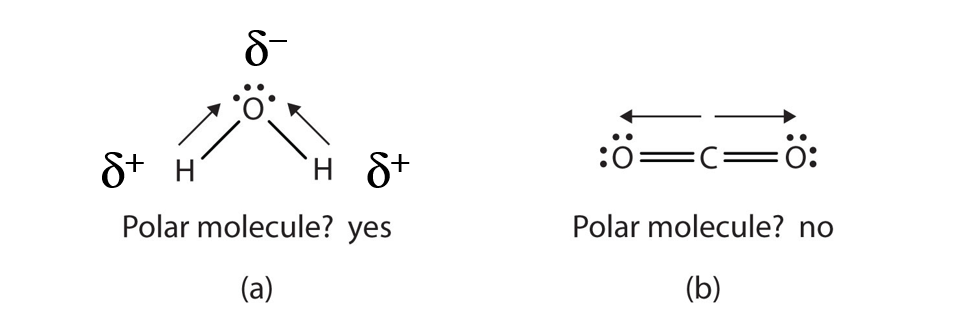
Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry
A molecule that is symmetrical in shape will have nonpolar bonds.
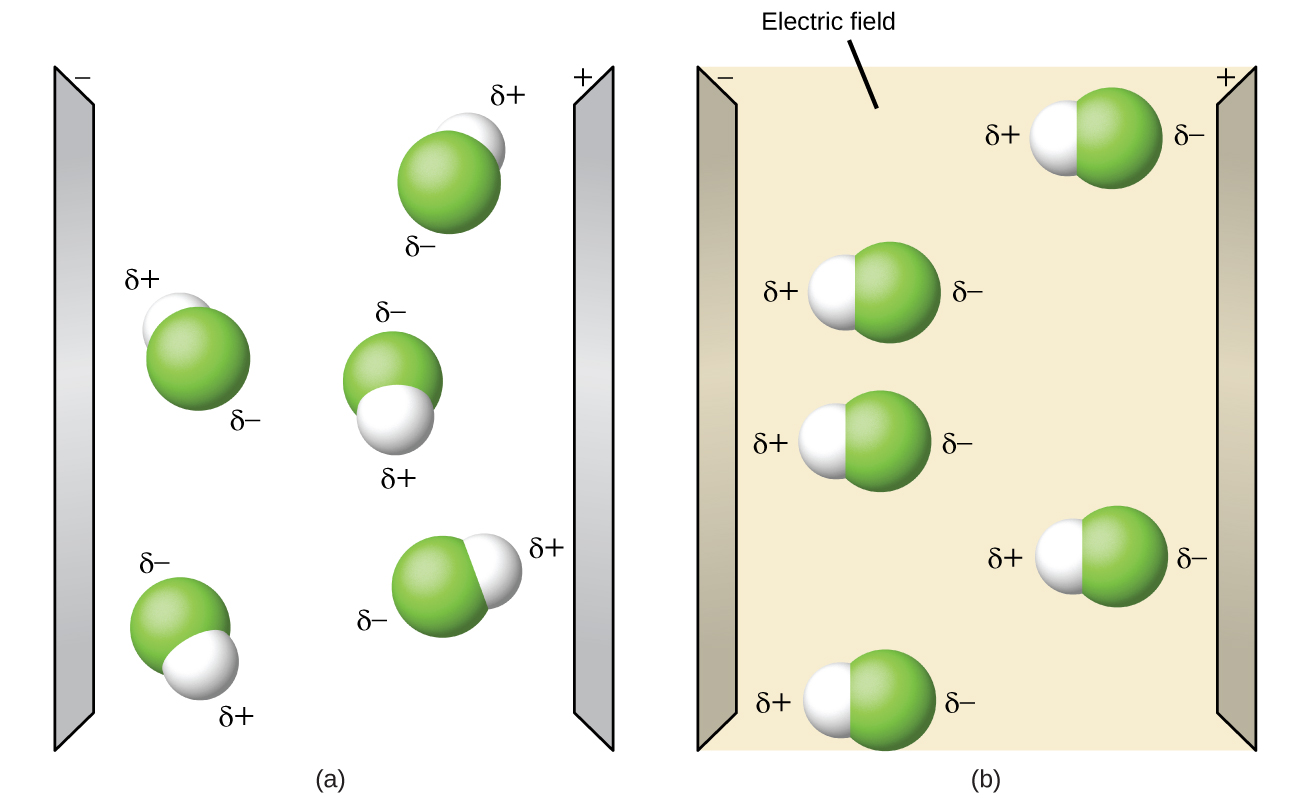
. If the electronegativity difference between the atoms is greater than 20 the bond is ionic. 34 Key Terms APEX Biology. A molecule with an unbalanced arrangement of charge where one end of the molecule is more positive or negative than the other end.
When the molecule contains polar bonds C. How to Determine the Polarity of a Molecule. The presence of both covalent and ionic bonds in the molecule.
When the molecule contains hydrogen bonds D. 33 Key Terms APEX Biology. If the electronegativity difference between the two atoms is between 05 and 20 the atoms form a polar covalent bond.
Molecules are made up of two or more atoms either of the same element or of two or more different elements. When the molecule contains nonpolar bonds 2 See answers Advertisement Advertisement HANicole HANicole Answer. That is quite a difficult question to answer without a specific example of a molecule.
Sometimes molecules are bonded in a way that unevenly distributes charge and creates 2 poles 1 positive and 1 negative. Advertisement Advertisement mastymiddy mastymiddy Answer. There is a symmetrical.
If the molecule contains atoms of similar size polarity will cancel out. Polar covalent bonds within the molecule that are not symmetrical. B When the molecule contains polar bonds.
Polar molecules occur when there is an electronegativity difference between the bonded atoms. There should be an icon on the top right of the Apex assignment in the 1st Study section of Chapter 3. The uneven bend in the bond between oxygen and hydrogen.
What makes a molecule polar. The more uneven the distribution the more polar the molecule. What makes water a polar molecule.
What kind of forces act between molecules. Ionic compounds such as common salt are made up not of molecules but of ions arranged in a crystalline structure. Nonpolar molecules occur when electrons are shared equal between atoms of a diatomic molecule or when polar bonds in a larger molecule cancel each other out.
A on polar molecule is when there is an equal sharing of electrons between the two atoms of a diatomic molecule. A polar molecule is a molecule in which one end of the molecule is slightly positive while the other end is slightly negative. Polar molecules occur when two atoms do not share electrons equally in a covalent bond.
A molecule with two poles is called a dipole see figure below. The smallest particle of a compound that has all the chemical properties of that compound. If the molecule is symmetrical the effects of polarity will cancel out.
52 Key Terms APEX Biology. Is ch2o polar or nonpolar or ionic. If the electronegativities of the peripheral bonds are equal and the bond lengths between the atoms are equal the chances are its non-polar.
The ionic bond between oxygen and hydrogen d. But I like this question because the answer can be wildly against common sense the enemy of science. As to the polarity a molecule is either polar or non-polar based on the difference in the electronegativity values of the atoms present and.
A molecule that has a symmetrical shape will be a nonpolar molecule. Hydrogen fluoride is a dipole. Answer 1 of 3.
A polar molecule is characterized by the uneven distribution of the electrons that form the covalent bonds between each atom in the molecule resulting in a slightly positively charged side and a slightly negatively charged side. When the sharing of electrons between two atoms is unequal the molecule is said to be polar and when the sharing of electrons between the atoms is equal the molecule is said to be non-polarFormaldehyde CH 2 O is a polar compound. Unlike ions molecules carry no electrical charge.
The presence of only nonpolar bonds in the molecule B. A polar molecule has to have 2 or more different atoms with different electronegativities sothat electron density shifts from equal distribution between 2 atoms in a bond to a greater density around the more electronegative element. The presence of Van der Waals forces C.
A molecule that is not symmetrical will be a nonpolar molecule. How can we make. The presence of a net charge that does not cancel out D.
Molecules are groups of atoms bonded together. However if there is an unequal distribution of electronegativity within the molecule the molecule will be polar. The equal forces between oxygen and hydrogen c.
A diatomic molecule that consists of a polar covalent bond such as HF is a polar molecule. What causes a molecule to have a net dipole moment. A molecule becomes polar when the elements form polar bonds--bonds with a slight negative and a slight positive charge--that are uneven due to the structure of the molecule.
The liquid nature of water b. What causes a molecule to have a net dipole moment. Choice When the molecule contains nonpolar bonds.
This occurs because of the differences in electronegativity between atoms of different elements. If the molecule contains more than two bonds shape will not affect polarity. 41 Key Terms APEX Biology.
When this happens the molecule is considered polar. 2b When does the shape of a molecule affect its polarity. If the molecule contains nonpolar bonds a bent shape will make it polar.
This is because of the unbalanced electron density.
What Is A Polar Molecule Quora

Molecular Structure And Polarity Chemistry For Majors
What Is A Polar Molecule Quora

How Can The Shape Of A Molecule Determine Its Polarity Brainly Com
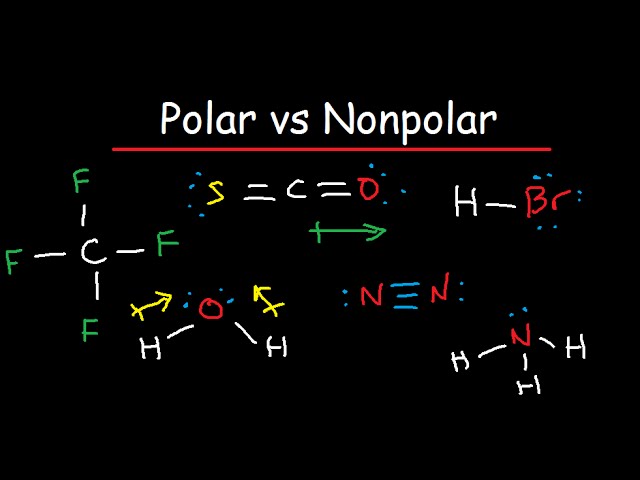
Polar And Nonpolar Molecules Is It Polar Or Nonpolar Youtube

7 6 Molecular Structure And Polarity Chemistry

Why Is Water A Polar Molecule Water Molecule Molecules Polarity Of Water
Is Sif4 A Polar Or A Non Polar Molecule Quora
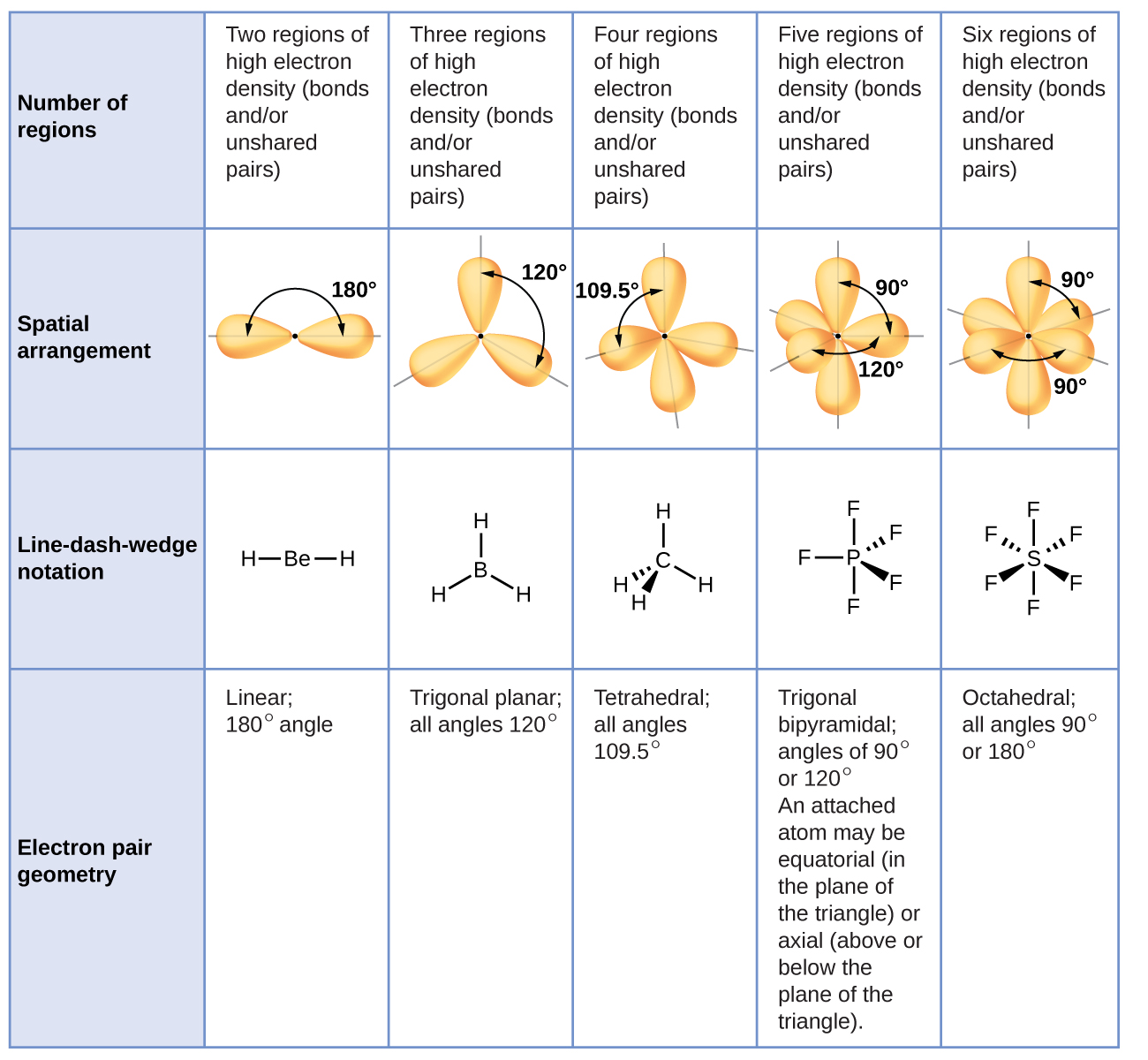
7 6 Molecular Structure And Polarity Chemistry

Is Nh3 Polar Or Nonpolar Quora

6 2 Molecular Shape And Polarity Chemistry Libretexts
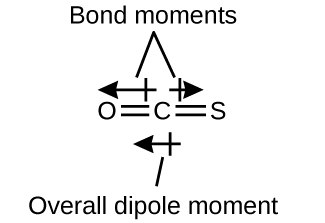
7 6 Molecular Structure And Polarity Chemistry
What Is A Polar Molecule Quora

Molecule Polarity Polarity Electronegativity Bonds Phet Interactive Simulations Teaching Chemistry Chemical Science Chemistry Education

What Makes A Molecule Polar Brainly Com
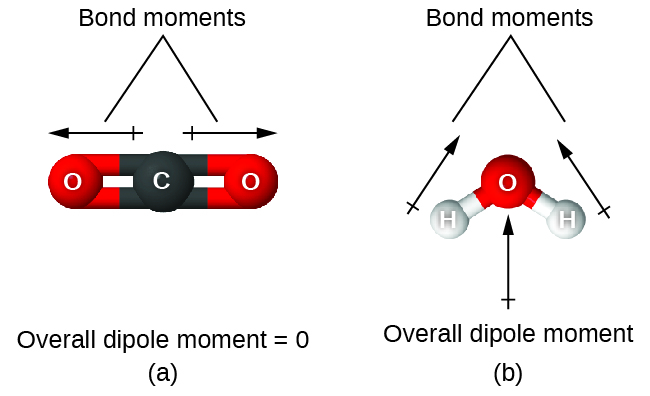
7 6 Molecular Structure And Polarity Chemistry Libretexts
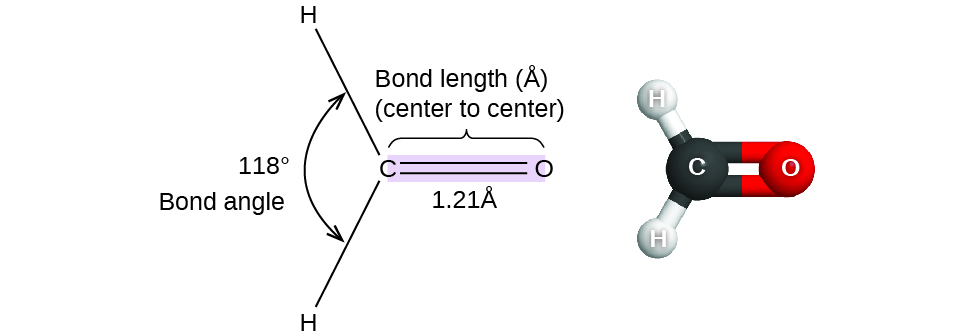
7 6 Molecular Structure And Polarity Chemistry
What Is A Polar Molecule Quora

Polar And Non Polar Covalent Molecules Polar Vs Nonpolar Youtube Playlist Science Chemistry Chemistry Science Activities
Comments
Post a Comment